Answer:
![\rm [H_3O^(+)] = 10^(-8.55)\;M\approx 2.82* 10^(-9) \;M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/3scuuhptymzy8r6pm5feyw9omskmdfxegj.png) , and
, and
![\rm [OH^(-)] = 10^(-5.45)\; M\approx 3.55* 10^(-6)\;M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/6mg6wqxlg4qhjbf57pxg13xzvkfbtr1koe.png)
This solution is likely to be basic.
Assumption: this solution is under room temperature, where
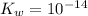 .
.
Step-by-step explanation:
The concentration of hydronium ions
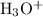 in the solution can be found from the
in the solution can be found from the
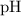 value. This relationship does not depends on temperature.
value. This relationship does not depends on temperature.
![\mathrm{[H_3O^(+)]} = 10^{-\mathrm{pH}} = 10^(-8.55)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/r94zo4yfjgpwfsncn89iu3fqe8ids4r1wc.png) .
.
The question states that for this solution,
![\rm [H_3O^(+)] = 8.55\;M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/bahvbkr95cr2y27rvxzr340tgvuj9utx6k.png) . Apply the relationship between
. Apply the relationship between
![\mathrm{[H_3O^(+)]}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/webs03kc6tnd1kth3woy2u6yqi1lymnepm.png) ,
,
![\mathrm{[OH^(-)]}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/g53pf4mjpy696zra8f5sps73aqhld01rl8.png) , and
, and
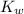 . Note that the value of
. Note that the value of
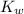 is dependent on the temperature of the solution.
is dependent on the temperature of the solution.
![\displaystyle \rm [OH^(-)] = \frac{\mathnormal{K_w}}{[H_3O^(+)]} = (10^(-14))/(10^(-8.55)) = 10^(-5.45)\; M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/yadppnvza7o9inhipen3ca2tgfml4i9uzh.png) .
.
In other words,
![\rm [H_3O^(+)] < [OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/5jwjbm9pot558h7zjw3chqoghknlu7ni19.png) .
.
In other words, this solution is basic.