Step-by-step explanation:
Since, it is given that density is 1.16 grams per milliliter and molar mass of copper sulfate pentahydrate is 249.68 g/mol.
Now, as we known that density is the amount of mass present in a unit volume.
Mathematically, Density =
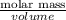
Hence, calculate the volume as follws.
Density =
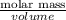
1.16 g/mL =
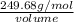
volume = 215.24 mL
As, 215.24 mL can dissolve in 249.68 g/mol of complex. So, in 100 mL volume amount dissolved will be calculated as follows.
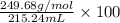
= 116 grams
Thus, we can conclude that 116 grams of copper sulfate pentahydrate that will dissolve in 100 g of water at 0
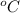 .
.