Step-by-step explanation:
It is known that atmospheric pressure is equal to 760 torr.
And, at atmospheric pressure that is, 760 torr the boiling point of pure water is 100 degree celsius.
So, calculate the boiling point of pure water at 653.7 torr as follows.
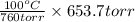
=
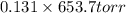
=
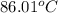
Therefore, we can conclude that the boiling point of pure water at 653.7 torr is
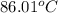 .
.