Answer:
0.6425 moles of
 is required to react with 25.7 grams of
is required to react with 25.7 grams of
 .
.
Step-by-step explanation:
mas of oxygen gas = 25.7 g
moles of oxygengas =
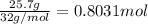
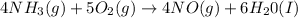
According to reaction given above, 5 moles of oxygen gas reacts with 4 moles of ammonia gas.
Then 0.8031 moles of oxygen gas will react with :
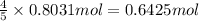 of ammonia gas
of ammonia gas
0.6425 moles of
 is required to react with 25.7 grams of
is required to react with 25.7 grams of
 .
.