Answer:
There are
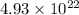 copper atoms in 5.2 gram of metallic copper.
copper atoms in 5.2 gram of metallic copper.
Explanation:
Start by finding the number of moles of copper atoms in that 5.2 gram of metallic copper. Look up the relative atomic mass of copper on a modern periodic table.
In other words, the mass of one mole of copper atoms is 63.546 gram.
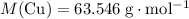 .
.
How many moles of copper atoms in that 5.2 gram sample?
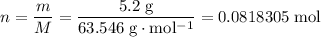 .
.
Now, how many atoms is
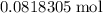 ?
?
The Avogadro's Number gives the number of particles in one mole:
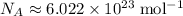 . (Encyclopedia Britannica)
. (Encyclopedia Britannica)
There are
 particles (a very large number) in one mole.
particles (a very large number) in one mole.
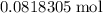 of copper atoms will thus contain
of copper atoms will thus contain
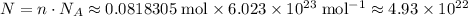
copper atoms.