Answer:
This solution is neutral.
Assumption: the solution is under room temperature, such that
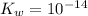 .
.
Step-by-step explanation:
For a solution in water,
![\mathrm{[H_3O^(+)] \cdot [OH^(-)]} = \mathnormal{K_w}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/fzs0m8dpzn1u7f3krfqe6yzusjjigah0g6.png) .
.
In other words, if
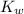 is given, knowing the concentration (in
is given, knowing the concentration (in
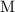 , or equivalently
, or equivalently
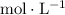 ) of either
) of either
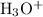 or
or
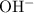 will imply the concentration of the other ion.
will imply the concentration of the other ion.
Under room temperature,
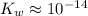 .
.
The question states that for this solution,
![\mathrm{[H_3O^(+)]} = \rm 1.00* 10^(-7)\; M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/lr545j65oe55gmeja4v1o3t5y5t6v9zvbf.png)
As a result, the concentration of
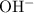 in this solution will be
in this solution will be
![\displaystyle \frac{K_w}{\mathrm{[H_3O^(+)]}} = (10^(-14))/(1.00* 10^(-7)) = 10^(-7)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/3w6h6x50328apm8xc9okgi1onu3hdja7gi.png) .
.
- A solution is acidic if
![\mathrm{[H_3O^(+)] > [OH^(-)]}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/1yq749os34kd8d74zbwqns9k0yppr9p2wb.png) .
. - A solution is basic if
![\mathrm{[H_3O^(+)] < [OH^(-)]}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vt9x23nmszsi33e3rv4codyuidrb7j4hz1.png) .
. - A solution is neutral if
![\mathrm{[H_3O^(+)] = [OH^(-)]}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/3enj4winkl100vgz9hsp47ma2hwli9u5ig.png) .
.
In this case,
![\rm [H_3O^(+)] = 10^(-7)\; M = [OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/6vlfw9wxm0hrpbf3kzocx16xndk9purggq.png) . In other words, this solution is neutral.
. In other words, this solution is neutral.