Answer:
Step-by-step explanation:
Given parameters:
Hydroxide ion concentration = 5.5 x 10⁻¹⁰M
Unknown:
concentration of hydroxonium ion [H₃O⁺]= ?
pH = ?
Solution
To determine the concentration of the hydroxonium ion, we know that from the ionic product of water:
[H₃O⁺][OH⁻]= 10⁻¹⁴
[H₃O⁺] =
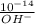
[H₃O⁺] =
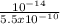
[H₃O⁺] = 1.8 x 10⁻⁵M
To find the pH of the solution, we use the expression below:
pH = -log₁₀[H₃O⁺]
pH = -log₁₀ 1.8 x 10⁻⁵ = -(-4.74) = 4.7
Since the pH of the solution is 4.7, it is acidic.