Answer:
The initial concentration of ethanal was 0.1590 mol/L.
Step-by-step explanation:
Integrated rate law for second order kinetic:
![k=(1)/(t)((1)/([A])-(1)/([A]_o))](https://img.qammunity.org/2020/formulas/chemistry/college/wzfhag8ps8rpawr3btb2e80cw2q8ub2hhe.png)
k = Rate constant =
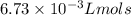
t = Time elapsed = 50.0 s
![[A]_o](https://img.qammunity.org/2020/formulas/chemistry/college/vfm07rqugmdqisio26k7clnl28e24vlfox.png) =initial concentration of ethanal
=initial concentration of ethanal
[A] = Concentration of ethanal left after time t = 0.151 mol/L
On substituting the value:
![6.73* 10^(-3) L mol s=(1)/(50.0 s)((1)/(0.151 mol/L)-(1)/([A_o]))](https://img.qammunity.org/2020/formulas/chemistry/college/xmk6oyp78b4ydl9pq251idlfv2dxe3cil7.png)
![[A]_o=0.1590 mol/L](https://img.qammunity.org/2020/formulas/chemistry/college/jx9u82obpbg4bru21t6j1hyt0kjvjdads4.png)
The initial concentration of ethanal was 0.1590 mol/L.