Answer:
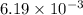 is the mole fraction of potassium dichromate.
is the mole fraction of potassium dichromate.
Step-by-step explanation:
Mass of potassium dichromate = 24.42 g
Moles of potassium dichromate =
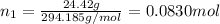
Mass of water = 240.0 g
Moles of water =
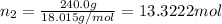
Mole fraction is calculated by:
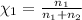
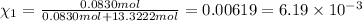
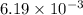 is the mole fraction of potassium dichromate.
is the mole fraction of potassium dichromate.