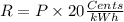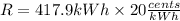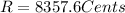Answer:
Part a)
P = 13.93 kW
Part b)
R = 8357.6 Cents
Step-by-step explanation:
Part A)
heat required to melt the aluminium is given by
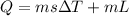
here we have
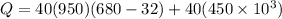
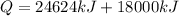
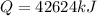
Since this is the amount of aluminium per hour
so power required to melt is given by
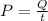
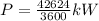

Since the efficiency is 85% so actual power required will be
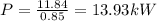
Part B)
Total energy consumed by the furnace for 30 hours
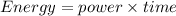
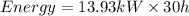
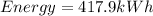
now the total cost of energy consumption is given as