Answer: There will be no change in rate.
Step-by-step explanation:
Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.

![Rate=k[A]^x[B]^y[C]^z](https://img.qammunity.org/2020/formulas/chemistry/college/ak6o97ujnkmg94pccpv7v8h0bf4d8snsqf.png)
k= rate constant
x = order with respect to A = 0
y = order with respect to B =
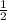
z= order with respect to C = 2
Thus
![Rate=k[A]^0[B]^{(1)/(2)}[C]^2](https://img.qammunity.org/2020/formulas/chemistry/college/sx8c1f5a0z793o9l8936i86ce30puqhzgp.png)
Given : when [A] is doubled and the other reactant concentrations are held constant.
Thus the new rate law is
![Rate'=k[2A]^0[B]^{(1)/(2)}[C]^2](https://img.qammunity.org/2020/formulas/chemistry/college/ey37hrgx9t2i30o2q3n1ngwmu2zf1bmn4q.png)
![Rate'=k[2]^0[A]^0[B]^{(1)/(2)}[C]^2](https://img.qammunity.org/2020/formulas/chemistry/college/48f31ewkoscyrmvni10grmqosksdglbc8e.png)
![Rate'=k[A]^0[B]^{(1)/(2)}[C]^2](https://img.qammunity.org/2020/formulas/chemistry/college/836r2bxfel6thp6znbh0kcunbxoxpbyxl9.png)
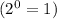
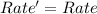
Thus the reaction rate would not change.