Step-by-step explanation:
According to ideal gas equation, product of pressure and volume equals the product of number of moles, gas constant and temperature.
Mathematically, PV = nRT
where P = pressure, V = volume
n = no. of moles, R = gas constant = 0.0821 atm L/mol
T = temperature
Since, it is known that number of moles equal mass divided by molar mass.
Hence, number of moles of given sample of acetone are as follows.
No. of moles =
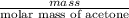
=
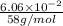
=
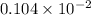 mole
mole
Therefore, putting the values in ideal gas equation as follows.
PV = nRT
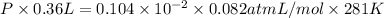
= 0.359 atm
In 1 atm equal to 760 mm Hg. So, convert 0.359 atm into mm Hg as follows.
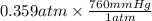
= 272.84 mm Hg
Hence, pressure of the ideal gas will be 100 mm Hg + 272.84 mm Hg = 372.84 mm Hg
Thus, we can conclude that the ideal gas pressure in the container if all of the liquid acetone evaporated is 372.84 mm Hg.