Answer:
1. Double displacement.
2. Combustion.
3. Simple displacement.
4.Double displacement.
Step-by-step explanation:
Hello,
1.
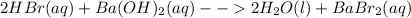
In this case, it is about a double displacement reaction since all the cations (H and Ba) and anions (Br and OH) are exchanged.
2.
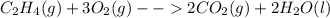
In this case, it is about the combustion of ethene.
3.
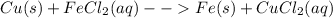
In this case, it is about a simple displacement reaction since the iron (II) cations become solid iron and on the contrary for copper.
4.

Finally, it is about a double displacement chemical reaction since the sodium and silver cations are exchanged with the sulfide and nitrate anions.
Best regards.