Answer : The mass of copper deposit is, 1.98 grams
Explanation :
First we have to calculate the charge.
Formula used :
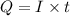
where,
Q = charge = ?
I = current = 10 A
t = time = 10 min = 600 sec (1 min = 60 sec)
Now put all the given values in this formula, we get
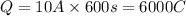
Now we have to calculate the number of atoms deposited.
As, 1 atom require charge to deposited =
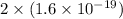
Number of atoms deposited =
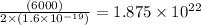 atoms
atoms
Now we have to calculate the number of moles deposited.
Number of moles deposited =
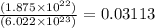 moles
moles
Now we have to calculate the mass of copper deposited.
1 mole of Copper has mass = 63.5 g
Mass of Copper Deposited =
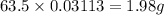
Therefore, the mass of copper deposit is, 1.98 grams