Answer:
1.23 J/(g °C)
Step-by-step explanation:
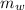 = mass of water = 50 g
= mass of water = 50 g
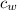 = specific heat of water = 4.186 J/(g °C)
= specific heat of water = 4.186 J/(g °C)
 = Initial temperature of water = 22.00 °C
= Initial temperature of water = 22.00 °C
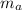 = mass of alloy = 25 g
= mass of alloy = 25 g
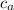 = specific heat of alloy = ?
= specific heat of alloy = ?
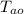 = Initial temperature of alloy = 93.00 °C
= Initial temperature of alloy = 93.00 °C
 = Final equilibrium temperature = 31.10 °C
= Final equilibrium temperature = 31.10 °C
Using conservation of heat
Heat Lost by alloy = Heat gained by water
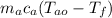 =
=
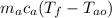
(25)
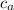 (93 - 31.10) = (50) (4.186) (31.10 - 22)
(93 - 31.10) = (50) (4.186) (31.10 - 22)
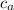 = 1.23 J/(g °C)
= 1.23 J/(g °C)