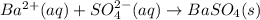Answer : The net ionic equation will be,
Explanation :
In the net ionic equations, we are not include the spectator ions in the equations.
Spectator ions : It is defined as the ions present on reactant and product side that do not participate in the reactions. That means the same ions present on both the sides.
The given balanced ionic equation will be,
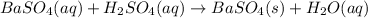
The ionic equation in separated aqueous solution will be,
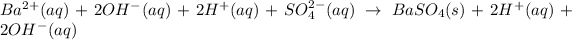
In this equation,
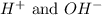 ions are the spectator ions.
ions are the spectator ions.
By removing the spectator ions from the balanced ionic equation, we get the net ionic equation.
The net ionic equation will be,