Answer:
Molar Mass of hemoglobin is
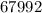 g/mol
g/mol
Step-by-step explanation:
As we know that
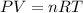
Where "P" signifies pressure, "V" signifies volume, "R" signifies gas constant, "T" signifies temperature and "n" signifies number of moles.
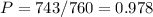 atm
atm
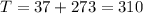 K
K
Substituting the given values in above equation, we get -
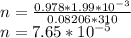 moles of oxygen
moles of oxygen
Number of moles of hemoglobin is
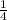 of moles of oxygen
of moles of oxygen
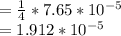 moles
moles
Molar mass of hemoglobin is equal to weight of hemoglobin divided by number of moles of hemoglobin
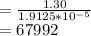 g/mol
g/mol