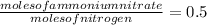Answer:
The conversion factor is 0.5, with which we have to multiply moles of nitrogen to convert it into moles of ammonium nitrate.
Step-by-step explanation:
As given the molecular formula of ammonium nitrate is NH₄NO₃
There are two nitrogen in each molecule.
thus in each mole of ammonium nitrate there are two moles of nitrogen present in it.
It means if there are two moles of nitrogen the mole of ammonium nitrate will be one
If there is one mole of nitrogen the moles of ammonium nitrate is 0.5.
So if we wish to convert moles of nitrogen to moles of ammonium nitrate we have to multiply the moles of nitrogen with 0.5.
The conversion factor is