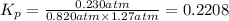Answer:
0.2208 is the equilibrium constant,
 .
.
Step-by-step explanation:
Equilibrium constant is defined as ratio of concentration of products to the concentration of reactants raised to the power equal to their stoichiometric coefficients in balanced chemical equation. It is expressed as

If the equilibrium is in gaseous phase then instead of concentration take partial pressure of each compound.The It is expressed as
 .
.
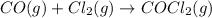
Partial pressure of the
![p_([CO]) = 0.820 atm](https://img.qammunity.org/2020/formulas/chemistry/college/8yo0dk7kha6gs921h0x5lljrk9ez8bevrr.png)
Partial pressure of the
![p_([Cl_2]) = 1.27 atm](https://img.qammunity.org/2020/formulas/chemistry/college/9i35dwt1nyrg6qgd1md83upibfm32t8g0w.png)
Partial pressure of the
![p_([COCl_2]) = 0.230 atm](https://img.qammunity.org/2020/formulas/chemistry/college/jko6ojjgma2ddv1vlkzll304r577f0wmwj.png)
The expression of an equilibrium constant is given as:
![K_p=(p_([COCl_2]))/(p_([CO])p_([Cl_2]))](https://img.qammunity.org/2020/formulas/chemistry/college/dw8f6roimdh7bopvpklhi83lh3o08lgbcb.png)