Answer:
The number of acetic acid moles is 0.0090 in 10 ml of the solution.
Step-by-step explanation:
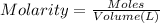
Let the moles acetic acid in vinegar be n.
Molarity of the vinegar solution = 0.90 M
Volume of the vinegar solution = 10 mL = 0.010 L
1 mL = 0.001 L
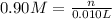
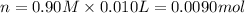
The number of acetic acid moles is 0.0090 in 10 ml of the solution.