Answer: No the given gas is not at STP.
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
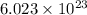 of particles.
of particles.
Standard condition of temperature (STP) is 273 K and atmospheric pressure is 1 atmosphere respectively.
Given temperature :
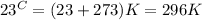
1 mole of every gas occupy volume at STP = 22.4 L
Thus at STP, the temperature is 273 K and pressure is 1 atmosphere and the given gas is not at STP.