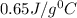Answer: The metal is Calcium.
Step-by-step explanation:
To calculate the specific heat of substance during the reaction.
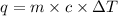
where,
q = heat absorbed = 789.75 J
c = specific heat of metal = ?
m = mass of substance = 18.0 g
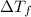 = final temperature - initial temperature =
= final temperature - initial temperature =
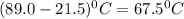
Now put all the given values in the above formula, we get:
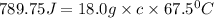
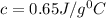
As specific heat is characteristic of each metal and thus the metal is calcium which has specific heat of