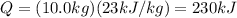Answer:
230 kJ
Step-by-step explanation:
The amount of heat needed to melt a substance at its melting point is:
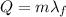
where
m is the mass of the substance
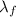 is the latent heat of fusion of the substance
is the latent heat of fusion of the substance
In this problem,
m = 10.0 kg is the mass of the lead sample
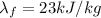 is the latent heat of fusion of lead
is the latent heat of fusion of lead
Substituting,