Answer:
Step-by-step explanation:
Remember that the atomic number of an element is the number of protons and the mass number is the number of protons plus neutrons.
1) Deuterium representation:
- Hydrogen: ⇒ H
- One proton: ⇒
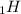
- One neutron: ⇒ add 1 to the mass number = 1 + 1 = 2 ⇒
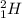
2) Helium-3 representation:
- He atom with 1 neutron: ⇒ mass number = 2 + 1 ⇒
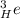
3) Neutron representation
- Atomic number 0 and mass number 1: ⇒
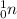
4) Nuclear equation:
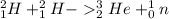 ← answer
← answer