Answer: Its pressure is also increased
Step-by-step explanation:
The expression for an Ideal Gas is:
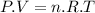
Where:
 is the pressure of the gas
is the pressure of the gas
 the number of moles of gas
the number of moles of gas
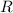 is the gas constant
is the gas constant
 is the absolute temperature of the gas
is the absolute temperature of the gas
As we can see, there is a direct proportional relation between the temperature and the pressure, which means that if the temperature increases the pressure of the gas increases as well.