Answer:
The number of protons 6.19 more than electron.
Step-by-step explanation:
Given that,
Charge
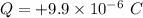
We know that,
formula of charge
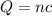
Where,
Q = total charge
n = number of protons
e = charge of electron
Put the value into the formula
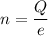
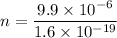
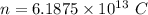
According to statement of question
Divide the answer by
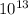
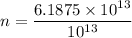
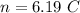
Hence, The number of protons 6.19 more than electron.