Answer:
95.3 grams.
Step-by-step explanation:
Relative atomic mass data from a modern periodic table:
How many moles of Na are consumed?
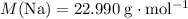 .
.
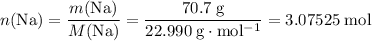 .
.
How many moles of
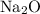 formula units will be produced?
formula units will be produced?
Consider the ratio between the coefficient of
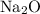 and that of
and that of
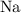 in the equation:
in the equation:
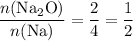 .
.
As a result,
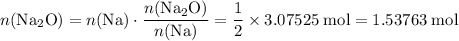 .
.
What will be the mass of that many
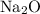 ?
?
Formula mass of
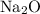 :
:
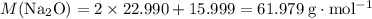 .
.
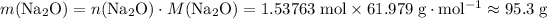 .
.