Answer:
The stoichiometric coefficient of aluminium is 2 that is option B is correct.
Step-by-step explanation:
Stoichiometric coefficient is the numeral coming or written before the symbol of compound or an element in balanced chemical reaction equation.
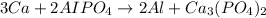
According to stoichiometry, 3 moles of calcium reacts with 2 moles of aluminum phosphate to give 2 moles of aluminium and 1 mole of calcium phosphate.
The stoichiometric coefficient of calcium is = 3
The stoichiometric coefficient of aluminum phosphate = 3
The stoichiometric coefficient of aluminium = 2
The stoichiometric coefficient of calcium phosphate = 1