Hello!
The answer is:
When the pressure that a gas exerts on a sealed container changes from
22.5 psi to 19.86 psi, the temperature changes from 110°C to
65.9°C.
Why?
To calculate which is the last pressure, we need to use Gay-Lussac's law.
The Gay-Lussac's Law states that when the volume is kept constant, the temperature (absolute temperature) and the pressure are proportional.
The Gay-Lussac's equation states that:
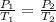
We are given the following information:
We need to remember that since the temperatures are given in Celsius degrees, we need to convert it to Kelvin (absolute temperature) before use the equation, so:
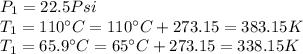
Now, calculating we have:
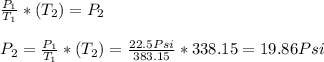
Hence, the final pressure is equal to 19.86 Psi.
Have a nice day!