Answer:
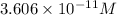 is the hydrogen ion concentration.
is the hydrogen ion concentration.
0.0002773 M is the hydroxide ion concentration.
The solution has pH value more than 7 which means that it is base.
Step-by-step explanation:
The pH of the solution is defined as negative logarithm of hydrogen ions concentration in a solution. Lower the value of pH more acidic will the solution.
Mathematically written as:
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
The pOH of the solution is defined as negative logarithm of hydroxide ions concentration in a solution. Lower the value of pOH more alkaline will the solution.
Mathematically written as:
![pOH=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/n477c3o3xy8p6ug3ipfjqh9fd53hdrrjyq.png)
The sum of ph and pOH is equal to 14.
pH + pOH = 14
We have:
Solution with pOH = 3.557
The pH of the solution = 14 - pOH = 10.443
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
![10.443=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/ypa93m8wx9sbvp03t1zyfcrt8eing465bq.png)
![[H^+]=3.606* 10^(-11) M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/nso70d7jvjesyow44h4ohkdp1gnf3g5iye.png)
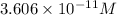 is the hydrogen ion concentration.
is the hydrogen ion concentration.
![pOH=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/n477c3o3xy8p6ug3ipfjqh9fd53hdrrjyq.png)
![3.557=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/wnjnriyqxc0yxbakr7azqhjhxvl3zm7akh.png)
![[OH^-]=0.0002773 M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/ilewbir5zw8v6163o2dm0izp2nunvc0r4w.png)
0.0002773 M is the hydroxide ion concentration.
The solution has pH value more than 7 which means that it is base.