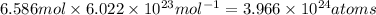Answer:
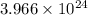 pure silver atoms are produced.
pure silver atoms are produced.
Step-by-step explanation:
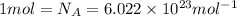
Atoms of copper =
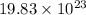
Moles of copper =
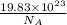
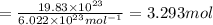
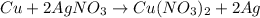
According to reaction, 1 mol of copper atoms gives 2 moles of silver atoms.
Then 3.293 moles of moles of copper will give:
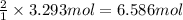
Number of silver atoms in 6.586 moles of silver:
=