Answer: The given statement is true.
Step-by-step explanation:
As the given molecule is
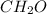 . Name of this compound is formaldehyde as it contains aldehyde functional group, that is, CHO group.
. Name of this compound is formaldehyde as it contains aldehyde functional group, that is, CHO group.
In this molecule, carbon is the central atom and both the hydrogen atoms are attached to the carbon atom through one single bond each.
Whereas oxygen is also attached to the carbon atom but through a double bond.
Therefore, we can conclude that the statement molecule
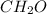 contains two single bonds and one double bond, is true.
contains two single bonds and one double bond, is true.