Answer:
Compound B.
Step-by-step explanation:
The freezing point depression is a colligative property. It depends on the number of particles (moles) present in the solution.
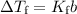
where b is the molal concentration
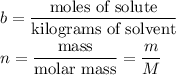
If m is constant (5 g), then
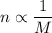
The compound with the greater molar mass has fewer moles and therefore fewer particles to depress the freezing point.
That must be Compound B, because Compound A has the lower freezing point.