Answer:
Both the shared electrons come from single atom
Step-by-step explanation:
Coordinate covalent bond is a type of bond in which both the shared pair of electrons are come from single bond. Whereas in simple covalent bond, sharing electrons come from both the participant atoms.
Coordinate covalent bond is also called dative bond and the atom which share both the electrons are called donar atom.
For a coordinate covalent atom to from, one participant atom should have lone pair of electron and other atom should be deprived of electrons.
In
 molecule, O atom has lone pair of electrons while H+ ions has deficiency of electrons, so O atom shares its lone pair of electrons with H+ ions which results in the formation of coordinate covalent bond and forms
molecule, O atom has lone pair of electrons while H+ ions has deficiency of electrons, so O atom shares its lone pair of electrons with H+ ions which results in the formation of coordinate covalent bond and forms
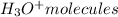 .
.