Answer:
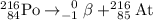 .
.
Both mass number (A) and atomic number (Z) shall conserve. Hence the mass number of the daughter nuclide shall be 216 and its atomic number be 85.
Let the mass number of the daughter nuclide be
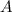 .
.
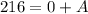 .
.
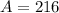 .
.
Let the atomic number of the daughter nuclide be
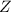 .
.
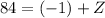 .
.
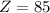 .
.
Look up the atomic number of the daughter nuclide in a modern periodic table. Alternatively, given that this reaction is a beta-minus decay, look for the element to the right of the parent nuclide.
Step-by-step explanation:
Nuclides are made of neutrons and protons.
- The atomic number (Z) is the number of protons in each of the nucleus.
- The mass number (A) is the number of protons plus neutrons in each of the nucleus.
The mass number shall thus be greater than or equal to the atomic number of a nucleus. However, note that occasionally the atomic number of electrons (beta-minus particles) is written as -1. The atomic number of positrons (beta-plus particles, the antiparticle of electrons) is written as +1. Beta particles do not contain protons or neutrons and might not satisfy rule
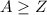 .
.
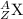 .
.
By convention
- The mass number of a nucleus is written at the upper-left corner of the atomic symbol.
- The atomic number of a nucleus is written at the lower-left corner of the atomic symbol.
Both mass number and atomic number shall conserve in nuclear reactions. In other words, the sum of all mass numbers on the left-hand side of a nuclear equation shall be the same as the sum of all mass numbers on the right-hand side. So is the case with atomic numbers. For this nuclear equation,
Mass number on the left-hand side:
- 216 for
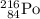 .
.
Mass numbers on the right-hand side:
- 0 for the beta-minus particle
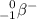 , and
, and - Let
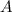 be the mass number of the daughter nuclide
be the mass number of the daughter nuclide
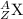 .
.
Mass numbers conserve. Therefore,
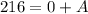 .
.
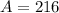 .
.
Similarly,
Atomic number on the left-hand side:
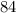 for
for
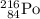 .
.
Atomic numbers on the right-hand side:
- "
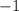 " for the beta-minus particle
" for the beta-minus particle
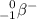 , and
, and - Let
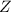 be the atomic number of the daughter nuclide
be the atomic number of the daughter nuclide
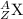 .
.
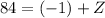 .
.
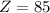 .
.
Refer to a modern periodic table. What is the element with the atomic number
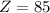 ? Astatine.
? Astatine.
Alternatively, notice that the atomic number
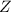 has increased by
has increased by
 relative to the parent nuclide
relative to the parent nuclide
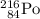 . Elements on a modern periodic table are order in the increasing order of atomic numbers. As a result, the daughter nuclide shall be on the right of the parent element.
. Elements on a modern periodic table are order in the increasing order of atomic numbers. As a result, the daughter nuclide shall be on the right of the parent element.
Complete the atomic symbol of the daughter nuclide:
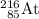 .
.