Answer:
60.42% is the percent yield of the reaction.
Step-by-step explanation:
Moles of methane gas at 734 Torr and a temperature of 25 °C.
Volume of methane gas = V = 26.0 L
Pressure of the methane gas = P = 734 Torr = 0.9542 atm
Temperature of the methane gas = T = 25 °C = 298.15 K
Moles of methane gas = n

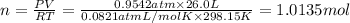
Moles of water vapors at 700 Torr and a temperature of 125 °C.
Volume of water vapor = V' = 23.0 L
Pressure of water vapor = P' = 700 Torr = 0.9100 atm
Temperature of water vapor = T' = 125 °C = 398.15 K
Moles of water vapor gas = n'
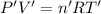
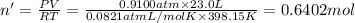
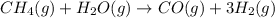
According to reaction , 1 mol of methane reacts with 1 mol of water vapor. As we can see that moles of water vapors are in lessor amount which means it is a limiting reagent and formation of hydrogen gas will depend upon moles of water vapors.
According to reaction 1 mol of water vapor gives 3 moles of hydrogen gas.
Then 0.6402 moles of water vapor will give:
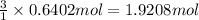 of hydrogen gas
of hydrogen gas
Moles of hydrogen gas obtained theoretically = 1.9208 mol
The reaction produces 26.0 L of hydrogen gas measured at STP.
At STP, 1 mole of gas occupies 22.4 L of volume.
Then 26 L of volume of gas will be occupied by:
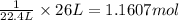
Moles of hydrogen gas obtained experimentally = 1.1607 mol
Percentage yield of hydrogen gas of the reaction:
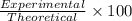

60.42% is the percent yield of the reaction.