Answer:
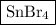
Step-by-step explanation:
The name tells you that this is a binary compound (contains two elements).
It contains a metal and a nonmetal, so it is a binary ionic compound. The general rule is:
Name of compound = name of metal name of ion (two words)
Name of metal = tin(IV), so the tin ion has a charge of 4+
Name of ion = bromide. Br is in Group 17, so bromide ion has charge of 1-.
