Answer : The correct option is, (B) bonding and unshared pairs around central atom.
Explanation :
Lewis-dot structure : It shows the bonding between the atoms of a molecule.
When we are determining the shape of a molecule then it is important to draw a Lewis-dot structure and for drawing a Lewis-dot structure we should know that how many total number of valance electrons present in a given compound or a molecule.
For example:
The given molecule is,
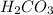
As we know that hydrogen has '1' valence electrons, carbon has '4' valance electrons and oxygen has '6' valence electron.
Therefore, the total number of valence electrons in
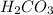 = 2 + 4 + 18 = 24
= 2 + 4 + 18 = 24
Now we have to determine the hybridization of carbon in
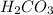
Number of bond pairs = 3
Number of lone pairs = 0
Number of atomic orbitals around carbon atom = 3 + 0 = 3
So, hybridization will be
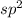 and the shape is, trigonal planar.
and the shape is, trigonal planar.
Hence, it is important to draw a Lewis Dot structure first in order to see the total number of bonding and unshared pairs around central atom.