Answer:
Approximately 1.9 kilograms of this rock.
Step-by-step explanation:
Relative atomic mass data from a modern periodic table:
To answer this question, start by finding the mass of Pb in each kilogram of this rock.
89% of the rock is
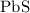 . There will be 890 grams of
. There will be 890 grams of
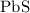 in one kilogram of this rock.
in one kilogram of this rock.
Formula mass of
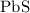 :
:
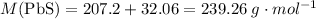 .
.
How many moles of
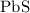 formula units in that 890 grams of
formula units in that 890 grams of
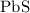 ?
?
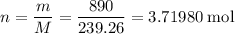 .
.
There's one mole of
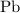 in each mole of
in each mole of
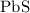 . There are thus
. There are thus
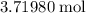 of
of
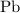 in one kilogram of this rock.
in one kilogram of this rock.
What will be the mass of that
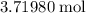 of
of
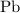 ?
?
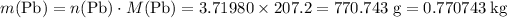 .
.
In other words, the
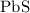 in 1 kilogram of this rock contains
in 1 kilogram of this rock contains
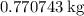 of lead
of lead
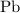 .
.
How many kilograms of the rock will contain enough
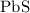 to provide 1.5 kilogram of
to provide 1.5 kilogram of
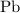 ?
?
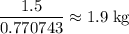 .
.