Answer:
0.004167 J/g°C is the specific heat of a substance.
Step-by-step explanation:
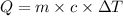
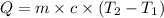
Where:
Q = heat absorbed(positive) or released (negative)
m = Mass of substance
c = specific heat of a substance
 = Initial temperature
= Initial temperature
 = Final temperature
= Final temperature
We have:
m =
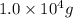
c = ? ,
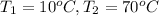
Q =
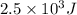
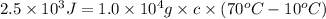
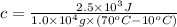
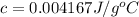
0.004167 J/g°C is the specific heat of a substance.