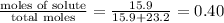Answer: a) percent by mass =
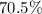
b) molality = 38.01mol/kg
c) mole fraction = 0.40
Step-by-step explanation:
Given molarity = 15.9 M
moles of
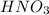 = 15.9 moles in 1.00 L of solution
= 15.9 moles in 1.00 L of solution
mass of
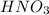 =
=
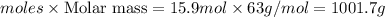
Density of solution of = 1.42 g/ml
Volume of solution = 1.00 L = 1000 ml
Mass of solution =
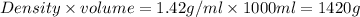
mass of solvent = mass of solution - mass of solute = (1420-1001.7) g = 418.3 g = 0.4183 kg
moles of solvent =
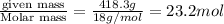
percent by mass =
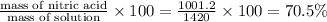
molality =
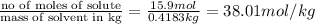
mole fraction =