Answer: The chemical reactions are given below.
Step-by-step explanation:
Combustion reaction is defined as the chemical reaction in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule.
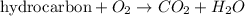
If supply of oxygen gas is limited, it is known as incomplete combustion and carbon monoxide gas is also produced as a product.
- For a: An excess of oxygen
Here, complete combustion reaction takes place. The chemical equation follows:
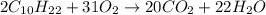
- For b: A slightly limited oxygen supply
Here, incomplete combustion takes place and carbon monoxide is also formed.
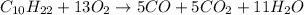
- For c: A very limited supply of oxygen
Here, incomplete combustion takes place and only carbon monoxide with water are formed as the products.
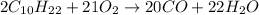
- For d: The compound is burned in air
When a compound is burned in air, it means that unlimited supply of oxygen is there. So, complete combustion reaction takes place and carbon dioxide gas is formed as a product.
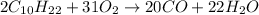
Hence, the chemical reactions are given below.