Answer: The correct answer is Option A.
Step-by-step explanation:
We are given:
4.5 wt % of Pb means that 4.5 grams of lead is present in 100 g of alloy.
95.5 wt % of Sn means that 95.5 grams of tin is present in 100 g of alloy.
To calculate the atom percent of any compound in a mixture, we use the equation:
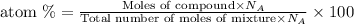
where,
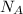 = Avogadro's number
= Avogadro's number
Moles of a compound is given by the formula:

Given mass of lead = 4.5 g
Molar mass of lead = 207.19 g/mol
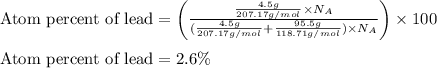
Given mass of tin = 95.5 g
Molar mass of tin = 118.71 g/mol
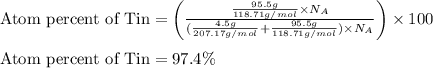
Hence, the correct answer is Option A.