Answer:
The molecules in the gas would be completely stationary
Step-by-step explanation:
The temperature of a gas is proportional to the average kinetic energy of its molecules, according to the equation (valid for an ideal monoatomic gas):
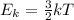
where
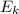 is the average kinetic energy of the molecules
is the average kinetic energy of the molecules
k is the Boltzmann constant
T is the absolute temperature of the gas
For a gas at 0 Kelvin, T = 0: this means that the average kinetic energy of the molecules is also zero. But this means that the motion of the molecules has completely stopped, so the molecules are completely still.