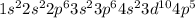Answer : The correct option is,
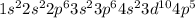
Explanation :
The given element bromine belongs to the group 17 and period 4. The symbol of bromine is, Br.
The atomic number of bromine = 35
The total number of electrons present in bromine element = 35
Electronic configuration : It is defined as the arrangement of electrons around the nucleus of an atom.
Hence, the correct electronic configuration of bromine is,