Answer:
a0=1
a1=6
a2=2
a3=3
Step-by-step explanation:
We have to start with the reaction:
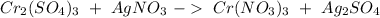
We have to start with "
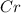 ". If we want to balance Cr we have to obtain 2 on both sides. Therefore we have to add a "2" on the right side, so:
". If we want to balance Cr we have to obtain 2 on both sides. Therefore we have to add a "2" on the right side, so:
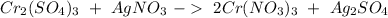
Then we can balance "
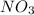 " as a whole. We have 6 on the right, we have to have the same amount on the left side, so:
" as a whole. We have 6 on the right, we have to have the same amount on the left side, so:
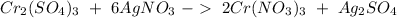
Then we can balance "
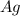 ". We have 6 on the left, so we have to have the same amount on the right if we already have 2 we have to put a · in front (on the right side) to obtain in total 6, so:
". We have 6 on the left, so we have to have the same amount on the right if we already have 2 we have to put a · in front (on the right side) to obtain in total 6, so:
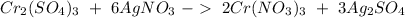
Finally, when we add this last number the
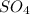 we will have 3 on both sides. Therefore the reaction is already balanced.
we will have 3 on both sides. Therefore the reaction is already balanced.