Answer: The correct answer is Option C.
Step-by-step explanation:
Water is a molecule which is formed by the combination of hydrogen and oxygen atoms. The chemical formula for this is

Ionization is a special type of dissociation process. It is defined as the process in which a molecules splits into its ions.
When water dissociates, it leads to the formation of 2 ions, which are hydroxide ion and hydrogen ion.
The chemical equation for ionization or dissociation of water molecule is:
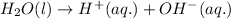
Hence, the correct answer is Option C.