Answer:
The electron
Step-by-step explanation:
The atom consists of three subatomic particles:
- The proton: protons are inside the nucleus of the atoms, they have positive charge of +e (
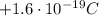 ), and a mass of approximately
), and a mass of approximately
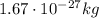
- The neutron: neutrons are also located inside the nucleus of the atoms, they have no electrical charge and mass similar to the mass of the proton
- The electron: electrons orbit around the nucleus in a cloud, they have negative charge of -e (
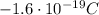 ), and their mass is about 1800 time smaller than the mass of the proton (
), and their mass is about 1800 time smaller than the mass of the proton (
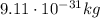 )
)