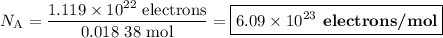Answer:
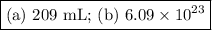
Step-by-step explanation:
(a) Gas produced at cathode.
(i). Identity
The only species known to be present are Cu, H⁺, and H₂O.
Only the H⁺ and H₂O can be reduced.
The corresponding reduction half reactions are:
(1) 2H₂O + 2e⁻ ⇌ H₂ + 2OH⁻; E° = -0.8277 V
(2) 2H⁺ +2e⁻ ⇌ H₂; E° = 0.0000 V
Two important points to remember when using a table of standard reduction potentials:
- The higher up a species is on the right-hand side, the more readily it will lose electrons (be oxidized).
- The lower down a species is on the left-hand side, the more readily it will accept electrons (be reduced}.
H⁺ is below H₂O, so H⁺ is reduced to H₂.
The cathode reaction is 2H⁺ +2e⁻ ⇌ H₂, and the gas produced at the cathode is hydrogen.
(ii) Volume
a. Anode reaction
The only species that can be oxidized are Cu and H₂O.
The corresponding half reactions are:
(3) Cu²⁺ + 2e⁻ ⇌ Cu; E° = 0.3419 V
(4) O₂ + 4H⁺ + 4e⁻ ⇌ 2H₂O E° = 1.229 V
Cu is above H₂O, so Cu is more easily oxidized.
The anode reaction is Cu ⇌ Cu²⁺ + 2e⁻.
b. Overall reaction:
Cu ⇌ Cu²⁺ + 2e⁻
2H⁺ +2e⁻ ⇌ H₂
Cu + 2H⁺ ⇌ Cu²⁺ + H₂
c. Moles of Cu lost
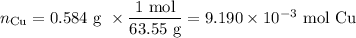
d. Moles of H₂ formed
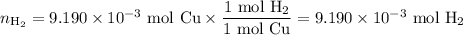
e. Volume of H₂ formed
Volume of 1 mol at STP (0 °C and 1 bar) = 22.71 mL
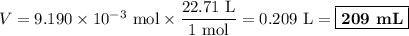
(b) Avogadro's number
(i) Moles of electrons transferred
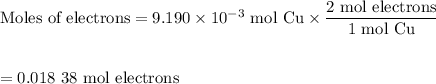
(ii) Number of coulombs
Q = It
Q = \text{1.18 C/s} \times 1.52 \times 10^{3} \text{ s} = 1794 C
(iii). Number of electrons
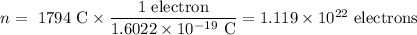
(iv) Avogadro's number