Answer : The Lewis-base in this reaction is, water

Explanation :
According to the Lewis acid-base theory,
Lewis acid : It is a substance that can accepts pairs of electrons to form a covalent bond.
Lewis-base : It is a substance that can donates pairs of electrons to form a covalent bond.
The given balanced equation is:
![Fe^(3+)+6H_2O\rightarrow [Fe(H_2O)_6]^(3+)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vvvigaak9qlby5w82346yu0y090gi5th0d.png)
In this reaction,
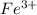 ion is Lewis-acid and
ion is Lewis-acid and
 is a Lewis-base. The
is a Lewis-base. The
 donates electrons to
donates electrons to
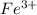 ion to form
ion to form
![[Fe(H_2O)_6]^(3+)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/epc67pz6kjvodrrsw30omm4gpsm5si00ro.png) .
.
Hence, the Lewis-base in this reaction is, water
